How to find ICSRs related to COVID-19 treatments in VigiLyze
UMC has created these guidelines to help users in the WHO Programme for International Drug Monitoring find adverse event reports linked to COVID-19 treatments. VigiLyze is updated weekly with an overview of the latest incoming data, and through VigiLyze you are also able to learn from the experience reported by other countries.
As described in “How to capture ICSRs for COVID-19 treatments”, the most important information to capture to retrieve all cases related to COVID-19 in VigiBase is indication. Unfortunately, indication is not yet searchable in VigiLyze, but we are exploring the possibility to add this feature.
Meanwhile, UMC provides an overview of all incoming data and reports are made available weekly in VigiLyze where we have used indication, but also other search parameters, to find relevant cases. You can also benefit from other countries’ experience with treatments related to COVID-19 by looking at their reporting in VigiLyze.
VigiLyze is updated every week and today over 80 countries share their data with VigiBase more often than once a month. To help you find COVID-19 related reports in VigiLyze, here are some points to consider.
For VigiFlow users see also: Using VigiFlow for data collection - COVID-19
Do you have the right focus?
VigiLyze is by default set to show your country data first. You can easily see all reports in VigiBase by clicking Global view. You can also easily expand your default region to include more countries or only have the Global view as your default view. To control your personal settings, use the menu in the top right corner.
If you’re collaborating with other countries, or if you know of other countries that have the same recommendations and approach for COVID-19 as your own, you can change the default region in your personal settings and add more countries. Or create a custom country group that you can use and re-use – this makes it easier to switch between having your own country in focus and the expanded region.
Use custom drug groups to easily retrieve all new cases for relevant drugs
It is possible to create a custom drug group in VigiLyze that your organisation can use and re-use as a search filter. In your personal settings you can create customised groups of drugs, countries and reactions. You could, for example, group the drugs used to treat COVID-19 in your country and then each week use VigiLyze to look for the latest reports of these drugs from other countries in the WHO programme.
Multi-ingredient drugs and multiple drugs given in combinations
To search for drugs with more than one active ingredient, like a fixed combination of lopinavir and ritonavir, simply type the first part of the ingredients’ name with a space in between in the search field (“lopin riton”) and select the Active ingredient Lopinavir;Ritonavir entry from the list. It doesn’t matter in which order you type the ingredients.
Still using the same example, this time we want to find cases with the combination lopinavir and ritonavir given as separate preparations. Search for them separately and then simply drag and drop one on top of the other to retrieve cases where both lopinavir and ritonavir were given. You can combine these approaches to retrieve cases with Lopinavir;Ritonavir or (Lopinavir AND Ritonavir).
Use the date filter to narrow down results
If you are only interested in new cases, you can use the filter VigiBase initial date found under Admin to focus on the period since your last search, or to narrow down the search to only cases received in 2020 for example. Note that the line listing in VigiLyze always shows the most recent cases first, so you may not need the date filter.
Search for reports from specific studies
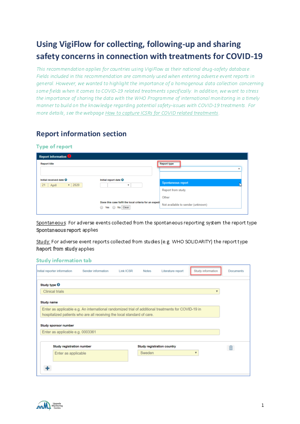
Cases reported with Study information are also searchable in VigiLyze. The Study name filter is available under Admin and will provide an as-you-type drop-down list of matching studies to choose from.
Filter for indication workaround: How to use Excel to filter for indication
VigiLyze results provide a graphic overview of cases, a line listing, disproportionality statistics and related investigations. Remember that the default view is your country (or region) and switching to Global view will give you the full picture.
Exporting the case line listing to Excel, one of the parameters you will get is drug indication. Filter for relevant indications (column Z) in the Cases tab using the text filter option with a “contains” search.
To retrieve the full case details for only cases with relevant indications, copy the UMC report ID (column A) for these reports and search for these specific reports again in VigiLyze using the UMC Report ID filter under Admin.
You have successfully worked your way to the cases that have a relevant indication and can now get a graphic overview of the characteristics of these cases.
Guidance on using VigiFlow
For guidance on how to best utilize VigiFlow in the COVID-19 context, read the document “Using VigiFlow for collecting, following-up and sharing safety concerns in connection with treatments for COVID-19” below.