Adverse event reporting in the palm of your hand
Matching WHO’s guidelines for reporting suspected side effects to medicines and vaccines, VigiMobile forms capture the data you need for analysis and informed decision-making.

Want to know more?
Write to us at
support@who-umc.org
VigiMobile forms have been developed together with WHO primarily for field reporting of suspected side effects from medicines and vaccines.
Health workers can use the forms to record adverse events from medicines and adverse events following immunisation (AEFI) anytime, anywhere on any device, even without internet. As soon as they are back online, they can send reports directly to VigiFlow or VigiFlow for AEFI for further investigation.
VigiMobile forms are simple and straightforward to use. Each form has its own URL, which you can distribute via a direct link, button on your website, or QR code. They appear on screen as a separate icon when they are downloaded so your reporters have easy access to them.
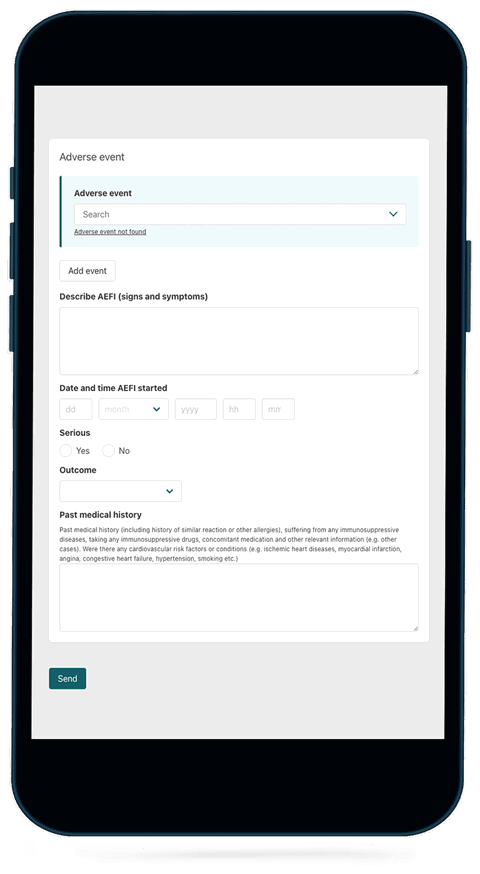
Based on WHO’s standard reporting forms for medicines and vaccines, VigiMobile forms capture all the core variables recommended by WHO for evaluating adverse event and AEFI data. Required fields and drop-down menus make data entry more efficient, reliable, and accurate. Structured reporting also solves the challenge of converting unstructured information into a format compatible with VigiFlow, speeding up the analysis process.

Key benefits
- Based on WHO’s standard reporting forms for medicines and vaccines, VigiMobile forms capture the core variables recommended by WHO for evaluating adverse event and AEFI data.
- Available free of charge to VigiFlow and VigiFlow for AEFI subscribers, VigiMobile forms can be installed on any device and on any operating system.
- VigiMobile forms simplify adverse event and AEFI reporting, capturing vital data in a fraction of the time that paper forms demand so that it is ready for you to work with.
- Reporters can use the forms even when they are not connected to the internet.
- The AEFI reporting form features drop-down menus containing prequalified vaccine lists and recognised adverse events for more efficient, reliable and accurate information gathering.
- Structured geographic data capture makes it possible to locate and investigate clusters of adverse events at national and subnational levels.
- Forms can be made available in your own language and customised with your logo and colour scheme on request.
- All information sent over the internet is encrypted keeping your data protected.
Got a licence?
Contact us to find out how you can get started at support@who-umc.org
Filling out forms on your phone or computer makes it much easier to report the right information. VigiMobile has now replaced paper reporting completely in my district.
Abdillah Mnenge City Council Pharmacist and AEFI Reporter, Tanga, Tanzania
Get the data you want, how you want it, when you want it
Unlike paper-based systems, where reporting delays, missing reports, a lack of relevant information, and human error can be problematic, digital forms increase the accuracy of the information you receive and give you the data you need to start working. This saves time and reduces your administrative burden by removing the need for manual data entry and verification.
Depending on your national safety surveillance system setup, you can configure workflows in VigiFlow to automatically route reports to regional or district authorities. With just one click, authorised users can easily share reports with VigiBase, the WHO global database of adverse event reports for medicines and vaccines.